Necrosis is a central pathophysiological driver of two major aspects during solid organ transplantation. First, it directly results in the loss of function of tubular cells (acute tubular necrosis, ATN). This process is mediated predominantly by ferroptosis. In parallel, vascular damage of peritubular capillaries contributes to damage. Second, necrotic cells release debris and pro-inflammatory cytokines. We hypothesize that antibody-mediated rejection, a clinically recognized condition that may lead to the loss of an allograft, may be triggered by pathways of regulated necrosis.
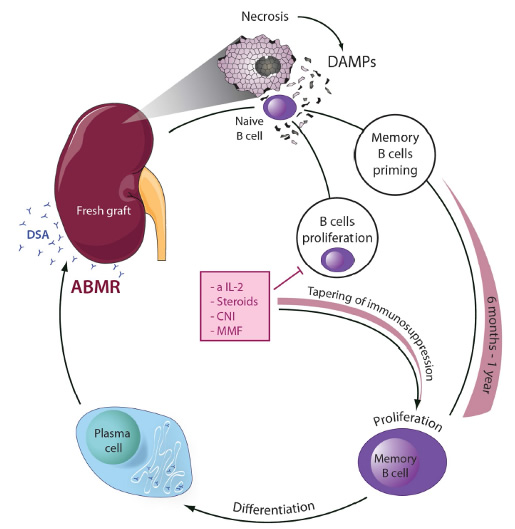
Upon transplantation of damaged (e.g. marginal) organs, necrotic debris is exposed to the immune system, e.g. naïve recipient B cells. DAMPs and cytokines, during the very first passage through the graft, are potently primed similar to a vaccination against the donor specific epitopes. Like a SARS-CoV2 vaccine, this process may be effective even in the presence of immunosuppression. Once immunosuppression is tapered, proliferative B cells may differentiate to plasma cells. Such plasma cells could produce de novo donor specific antibodies and potentially mediate ABMR. This model suggests that early interference with regulated necrosis possesses the capacity to prevent memory B cell priming and ABMR. Cell death inhibiting agents, e.g. small molecules added to the machine perfusate, are predicted to prevent ABMR. Find the PubMed link to our publications regarding transplantation here. We refer to the idea that necrotic cells drive a specific immune response as necroinflammation. Recently, we generated Nec-1f, a combined inhibitor of necroptosis and ferroptosis.